Gene Editing: A Single Dose
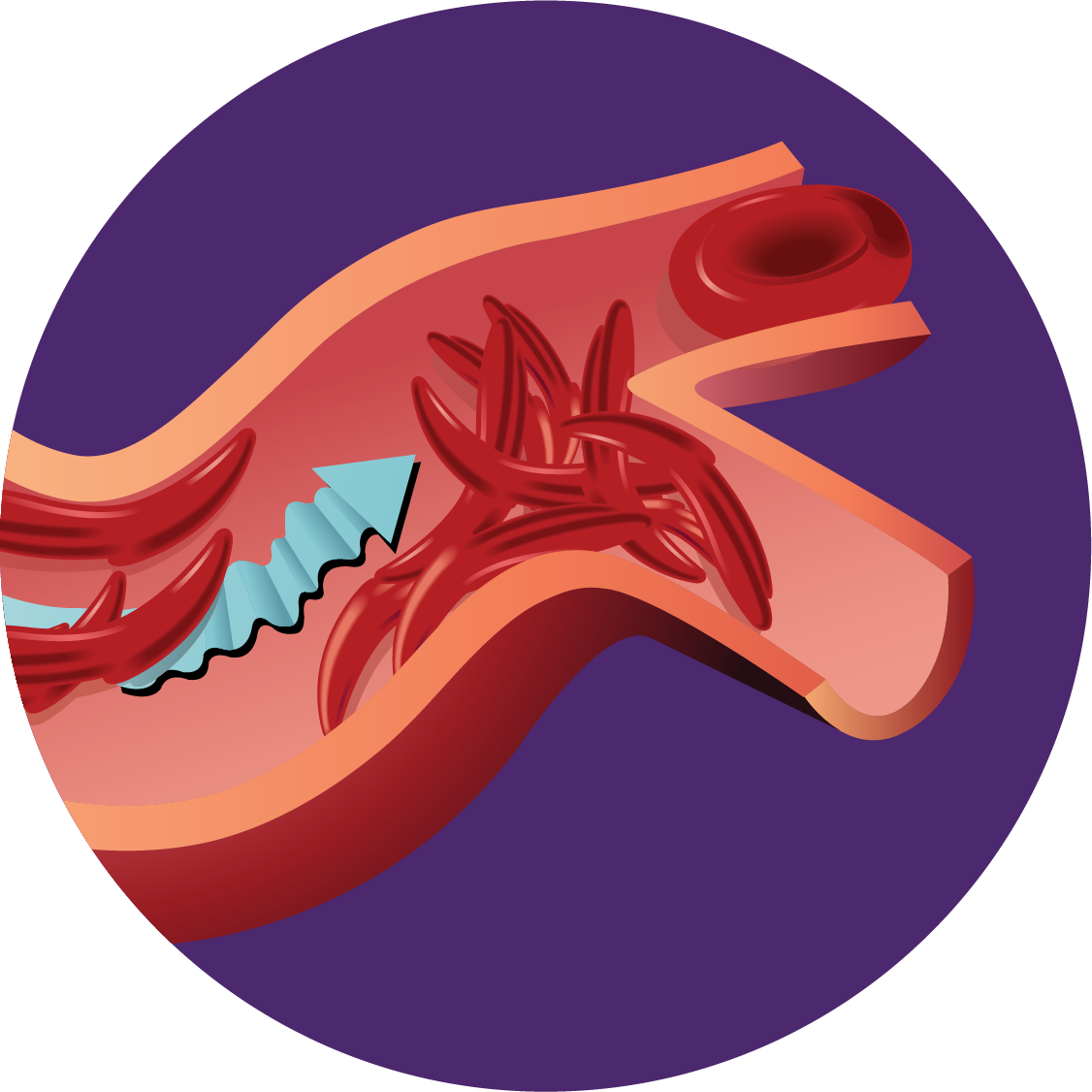
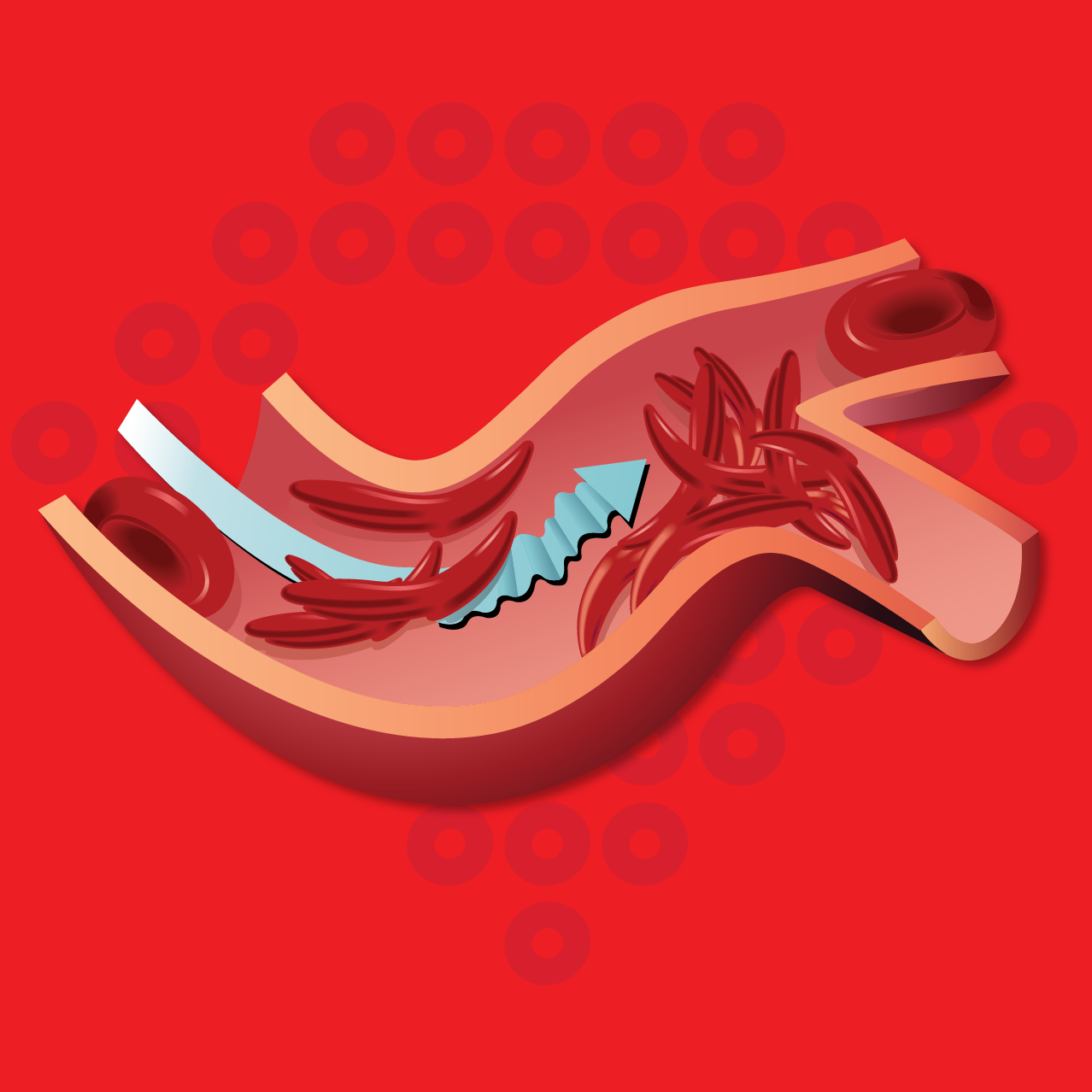
People living with SCD have an abnormal type of hemoglobin, called hemoglobin S, in their red blood cells. Hemoglobin S causes red blood cells to sickle which can block blood flow causing pain and damage to the tissues and organs, and ongoing symptoms.
Gene Editing with EDIT-301
Stem cells are collected from your bloodstream through your vein

Your own, edited stem cells are put back into your bone marrow where they can potentially create healthy, non-sickled blood cells in your body
RUBY Study Locations
Is gene editing different than gene therapy?
Gene editing is a type of gene therapy. Gene therapy includes addition, inhibition (stopping), editing, or replacement of a gene for disorders caused by a gene that is not working properly or is missing completely. Gene editing refers to cutting and modifying (editing) DNA (the genetic material in all cells) in your blood stem cells after they are collected from you.
Is gene editing the same as bone marrow transplant?
For SCD, bone marrow transplant (BMT) requires finding a suitable donor, while gene editing uses your own cells, eliminating the risk of graft-versus-host disease (GvHD). With BMT, patients may need drugs to suppress the immune system to avoid rejection, whereas these medications are not needed with gene therapy because patients are receiving their own modified cells.
With both procedures, the chemotherapy used to clear space for the new stem cells carries a risk of many long-term complications, which you should discuss with your doctor. Neither procedure impacts germ line cells, which means that SCD can still be passed on to biological children in the future.
Is RUBY a good fit for me?
To participate in RUBY, you must:
RUBY might not be a good fit for me if:
- Have an HLA-matched donor available
- Have had a previous stem cell transplant or are not able to receive an autologous transplant (with your own cells)
- Cannot tolerate any of the medications used in the trial
- Are unable to receive red blood cell transfusion
The study doctors will explain all the requirements to you.
Participation in this study is at no cost to you, including travel, meals, and any required accommodation for you and a companion.

“I am a sickle cell warrior. I was born with this debilitating disease, and although my life has been filled with pain, I’ve battled through the pain to be a relentless advocate. I’m hopeful for what the future holds for sickle cell disease and the breakthroughs in treatment we’ll see from clinical studies. I won’t let sickle cell be the boss.”
—Dima, living with sickle cell
What to Expect in the RUBY Study
Participating in the RUBY study has 3 phases: about 6 months to get ready, 5 weeks to receive the new investigational medicine, and up to 2 years of follow-up visits. During this time, you will be treated by a study doctor, and will remain connected to your regular SCD doctor.
The RUBY study will take place in multiple locations across the United States and Canada.

Part 1: Screening
Timing: about 6 months
Part 2: Administration
Timing: about 5 weeks

“Each of us living with SCD should consider participating in a clinical study. We are at an important time where many new technologies could offer the potential for a better life. These studies can be an important step toward learning more about sickle cell disease to help ourselves and our community.
Let’s do our part to see what we can unlock for SCD. I won’t let sickle cell be the boss.”
—Stephanie, sickle cell survivor
Connect with the RUBY team to discuss the study
It’s your call. If you are interested in learning more about the RUBY study, connect with us:
Call
Talk to a real person from the Medical Affairs team at Editas. Call 617-401-9007
Send an email ([email protected]) and we can followup with you.
Eligible?
Click here to review the list to see if you or someone you love might be eligible for the RUBY study. Submit your information if you would like us to follow up with you.